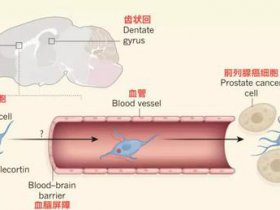
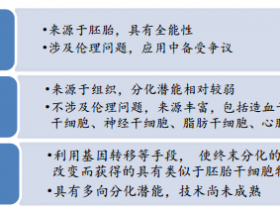
1、Cell Stem Cell:衰老相关神经发生衰退机制
2021年2月24日,来自瑞士苏黎世大学Sebastian Jessberger和美国威斯康星大学麦迪逊分校Darcie L. Moore研究组合作在《细胞-干细胞》上发表了题为“Declining lamin B1 expression mediates age-dependent decreases of hippocampal stem cell activity.”的研究论文,发现粘连蛋白B1(LB1)表达下降介导海马干细胞活性的衰老依赖性下降。
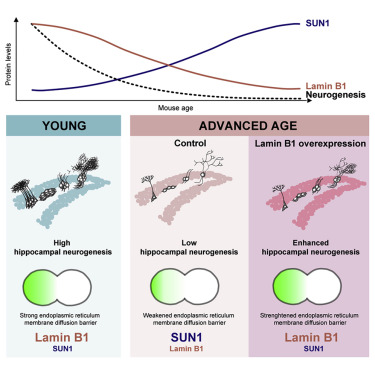
Fig 1|来源: Cell Stem Cell
他们表明,小鼠海马神经干细胞(NSC)中的核纤层蛋白层LB1随着年龄的增长而下调,而先前与Hutchinson-Gilford早衰综合征(HGPS)牵连的含SUN结构域的蛋白1(SUN1)的蛋白水平却增加了。平衡衰老的NSC中LB1和SUN1的水平可恢复内质网扩散屏障的强度,这与增殖的NSC中衰老因子的分离有关。
衰老NSCs中基于病毒的LB1表达的恢复增强了体外干细胞的活性,并增加了体内祖细胞的增殖和神经发生。因此,他们在这里确定了介导哺乳动物海马体中与衰老相关的神经发生衰退的机制。
据悉,NSC在整个海马齿状回中产生神经元。随着年龄的增长,神经发生水平急剧下降,这与海马记忆功能的下降有关。但是,介导NSC活性与年龄相关变化的细胞内在机制仍然未知。
Highlights
•Lamin B1 is downregulated with age in neural stem cells (NSCs)
•SUN1 protein levels increase in aged NSCs•Lamin B1 and SUN1 modulate ER membrane barrier strength
•Balancing lamin B1 expression enhances neurogenesis in the aged hippocampus
SummaryNeural stem cells (NSCs) generate neurons throughout life in the hippocampal dentate gyrus. With advancing age, levels of neurogenesis sharply drop, which has been associated with a decline in hippocampal memory function. However, cell-intrinsic mechanisms mediating age-related changes in NSC activity remain largely unknown. Here, we show that the nuclear lamina protein lamin B1 (LB1) is downregulated with age in mouse hippocampal NSCs, whereas protein levels of SUN-domain containing protein 1 (SUN1), previously implicated in Hutchinson-Gilford progeria syndrome (HGPS), increase. Balancing the levels of LB1 and SUN1 in aged NSCs restores the strength of the endoplasmic reticulum diffusion barrier that is associated with segregation of aging factors in proliferating NSCs. Virus-based restoration of LB1 expression in aged NSCs enhances stem cell activity in vitro and increases progenitor cell proliferation and neurogenesis in vivo. Thus, we here identify a mechanism that mediates age-related decline of neurogenesis in the mammalian hippocampus.
(评论: 重新激活衰老干细胞还是蛮有希望的。)
文章来源:
Muhammad Khadeesh bin Imtiaz, Baptiste N. Jaeger et al,Declining lamin B1 expression mediates age-dependent decreases of hippocampal stem cell activity,DOI: 10.1016/j.stem.2021.01.015,Cell Stem Cell:最新IF:21.464
2、Cell: 新型骨细胞有望成为骨质疏松症治疗的靶标
2021年2月24日,来自澳大利亚Michelle M. McDonaldWeng Hua Khoo合作在杂志cell上发表了题为“Osteoclasts recycle via osteomorphs during RANKL-stimulated bone resorption.”的研究结果,发现了一种新型细胞osteomorphs,望成为骨质疏松症治疗的靶标。
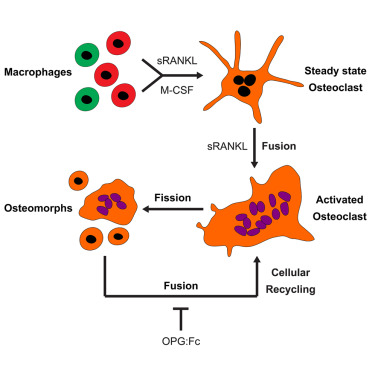
Fig 2|来源: Cell
破骨细胞由其单核前体细胞/巨噬细胞通过多种方式融合形成巨大的多核细胞,一旦吸收完成,就认为它们会发生凋亡。研究人员通过活体成像技术揭示了RANKL刺激的破骨细胞具有另一种细胞命运,其中它们分裂为称为骨形态的子代细胞osteomorphs。抑制RANKL阻止了这种细胞再循环并导致骨形态积聚。单细胞RNA测序显示,骨形态在转录上不同于破骨细胞和巨噬细胞,并表达许多在小鼠中缺失时与结构和功能性骨表型相关的非规范性破骨细胞基因。此外,人类骨形态基因直系同源基因的遗传变异会导致单基因骨骼疾病,并与骨矿物质密度有关,多基因骨骼特征。因此,破骨细胞通过骨形态回收,骨形态是一种参与骨吸收调节的细胞类型,可能是治疗骨骼疾病的靶标。
Highlights
•Osteoclasts fission into daughter cells called osteomorphs
•Osteomorphs fuse and recycle back into osteoclasts
•Osteomorph upregulated genes control bone structure and function in mice•Osteomorph upregulated genes are implicated in rare and common bone diseases in humans
SummaryOsteoclasts are large multinucleated bone-resorbing cells formed by the fusion of monocyte/macrophage-derived precursors that are thought to undergo apoptosis once resorption is complete. Here, by intravital imaging, we reveal that RANKL-stimulated osteoclasts have an alternative cell fate in which they fission into daughter cells called osteomorphs. Inhibiting RANKL blocked this cellular recycling and resulted in osteomorph accumulation. Single-cell RNA sequencing showed that osteomorphs are transcriptionally distinct from osteoclasts and macrophages and express a number of non-canonical osteoclast genes that are associated with structural and functional bone phenotypes when deleted in mice. Furthermore, genetic variation in human orthologs of osteomorph genes causes monogenic skeletal disorders and associates with bone mineral density, a polygenetic skeletal trait. Thus, osteoclasts recycle via osteomorphs, a cell type involved in the regulation of bone resorption that may be targeted for the treatment of skeletal diseases.
(评论: 不仅帮助我们了解了骨骼生物学,而且为骨质疏松症治疗提供了重要的新途径)
文章来源:
Michelle M. McDonald, Weng Hua Khoo et al,Osteoclasts recycle via osteomorphs during RANKL-stimulated bone resorption ,DOI:https ://doi.org/10.1016/j.cell.2021.02.002
3、Cell:揭示为什么克隆性造血会引发心血管疾病
2021年2月25日,来自美国哈佛医学院Kamila Naxerova研究组合作在《细胞》杂志在线发表了题为“Increased stem cell proliferation in atherosclerosis accelerates clonal hematopoiesis.”的研究成果,发现动脉粥样硬化中干细胞增殖的增加加速了克隆性造血作用。
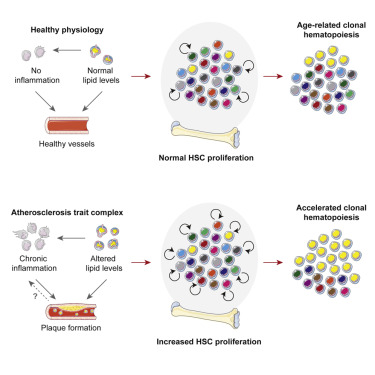
Fig 3|来源cell
研究人员发现,在患有动脉粥样硬化的小鼠和人类中,造血干细胞的分裂速率增加。数学分析表明,增加的干细胞增殖可加速具有驱动程序突变的克隆的体细胞进化和扩增。实验表明,动脉粥样硬化患者的细胞分裂率升高足以使到70岁时的克隆性造血风险增加3.5倍。
通过证明在长期升高的造血活动条件下,竞争性移植的Tet2-/-细胞的扩增会加速,研究人员证实了理论框架在动脉粥样硬化和睡眠破碎小鼠模型中的准确性。因此,造血干细胞增殖的增加是促成心血管疾病与克隆性造血之间联系的重要因素。
Highlights
•HSC proliferation is elevated in mice and humans with atherosclerosis
•Increased proliferation accelerates somatic evolution and clonal hematopoiesis (CH)
•Measured rates imply several-fold increased risk of CH in atherosclerosis patients•Mutant expansion is accelerated in mice with increased HSC proliferation
SummaryClonal hematopoiesis, a condition in which individual hematopoietic stem cell clones generate a disproportionate fraction of blood leukocytes, correlates with higher risk for cardiovascular disease. The mechanisms behind this association are incompletely understood. Here, we show that hematopoietic stem cell division rates are increased in mice and humans with atherosclerosis. Mathematical analysis demonstrates that increased stem cell proliferation expedites somatic evolution and expansion of clones with driver mutations. The experimentally determined division rate elevation in atherosclerosis patients is sufficient to produce a 3.5-fold increased risk of clonal hematopoiesis by age 70. We confirm the accuracy of our theoretical framework in mouse models of atherosclerosis and sleep fragmentation by showing that expansion of competitively transplanted Tet2 −/− cells is accelerated under conditions of chronically elevated hematopoietic activity. Hence, increased hematopoietic stem cell proliferation is an important factor contributing to the association between cardiovascular disease and clonal hematopoiesis.
(评论: 克隆性造血是一种个体造血干细胞克隆产生不成比例的血液白细胞的状态,这与心血管疾病的较高风险相关。但这种关联背后的机制尚不完全清楚。)
文章来源:
Alexander Heyde, David Rohde et al, Increased stem cell proliferation in atherosclerosis accelerates clonal hematopoiesis. DOI: 10.1016/j.cell.2021.01.049, Cell:最新IF:36.216