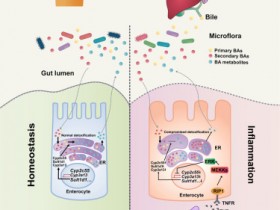
英夫利昔单抗(Infliximab)与抗Infliximab抗体:
英夫利昔单抗(Infliximab)是一类抑制TNFα的药物,主要用于克罗恩病, 类风湿关节炎, 强直性脊柱炎,牛皮癣关节炎,溃疡性结肠炎的缓解和治疗。抗TNFα治疗的临床疗效通常与治疗性抗体的谷浓度相关,即下一次应用抗TNFα抗体之前的药物浓度。有几个因素影响谷浓度,其中包括抗TNFα拮抗剂输注的剂量和频率、疾病活动度、个体药代动力学和免疫反应(形成抗药物抗体,ADA)。人们认为,ADA在功能性上中和了治疗性抗体或诱导其快速消除。ADA形成的后果可能是治疗失败和抗TNFα抗体应用期间的过敏反应。
检测的意义:
检测抗英夫利昔单抗(Infliximab)药物水平可帮助监测药物水平(尤其是谷浓度),以确保药物在循环中有足够的浓度,并在必要时调整其剂量。此外,在治疗过程中,谷浓度降低表明存在药物抗体。
如果患者体内产生抗英夫利昔单抗(Infliximab)抗体,则使用抗体药的疗法会导致无用的免疫反应。这可能导致过敏反应,导致疗效降低以及治疗失败。对抗英夫利昔单抗(Infliximab)抗体水平的监测有利于早期干预(例如,增加药物剂量或频率,增加给药免疫抑制药物,切换另一种药物),从而实现有效治疗,降低副作用。
在这里艾美捷为大家提供知名的诊断试剂和服务供应商Immundiagnostik(IDK)公司研发的爆款产品——抗英夫利昔单抗(Infliximab)药物水平检测试剂盒和抗英夫利昔单抗(Infliximab)ADA水平检测试剂盒::

相关产品推荐:
| 产品名称: | 英夫利昔(Infliximab)单抗药物浓度检测试剂盒 | 总抗英夫利昔(Infliximab)单抗抗体检测试剂盒 | 游离抗英夫利昔(Infliximab)单抗抗体检测试剂盒 |
| 货号: | K 9655 | K 9654 | K 9650 |
| 规格: | 96T | 96T | 96T |
| 孵育时间: | 1h/1h/10-20min | 20min/1h/1.5h/10-20min | 过夜/1h/10-20min |
| 样本类型 | 血清、EDTA血浆 | 血清、EDTA血浆 | 血清、EDTA血浆 |
| 上样量 | 10µl | 25µl | 50µl |
| 灵敏度 | LoB(空白限)= 1.998ng/ml | LoB(空白限)= 3.12 AU/ml | LoB(空白限)= 5.751 AU/ml |
| 校准品 | 4.15-225ng/ml |
*以上产品仅用于科研,不得做医疗及诊断用途
储存条件及有效期:
本产品在2-8℃下保存可稳定至所标示的有效期。
工作洗液配制后须储存于密封容器中,在2-8℃下可保存1个月。
结合物稀释后不稳定,无法保存,须现配现用。
生产日期及失效日期见试剂盒标签。
适用仪器:适用于具有450nm、620nm波长的所有全自动、半自动酶标仪。
样本类型:EDTA血浆和血清
样本保存:
新鲜采集的EDTA血浆或血清在室温(15-30℃)或2-8℃下可保存7天,若须长期保存,请置于-20℃下保存。
已稀释的EDTA血浆或血清样本在室温下可保存7天,在2-8℃可保存15天,在-20℃至少可保存7周。应尽量避免反复冻融,冻融次数不超过3次
K 9655部分引文:
- Afif W, Loftus E V, Faubion WA, Kane S V, Bruining DH, Hanson KA, et al. Clinical utility of measuring infliximab and human anti-chimeric antibody concentrations in patients with inflammatory bowel disease. The American journal of gastroenterology. 2010/02/11. 2010 May;105(5):1133–9.
- Beglinger C, Binek J, Braegger C, Michetti P, Rogler G, Sauter B, et al. InfliximabMonotherapie versus Kombinationstherapie mit Immunmodulatoren. The medical journal. 2008;1:32–4.
- Bender NK, Heilig CE, Dröll B, Wohlgemuth J, Armbruster F-P, Heilig B. Immunogenicity, efficacy and adverse events of adalimumab in RA patients. Rheumatology international. 2006/09/29. 2007 Jan 11;27(3):269–74.
- Bendtzen K, Geborek P, Svenson M, Larsson L, Kapetanovic MC, Saxne T. Individualized monitoring of drug bioavailability and immunogenicity in rheumatoid arthritis patients treated with the tumor necrosis factor alpha inhibitor infliximab. Arthritis and rheumatism. 2006 Dec;54(12):3782–9.
- Bradley JR. TNF-mediated inflammatory disease. The Journal of pathology. 2008 Jan;214(2):149–60.
- St Clair EW, Wagner CL, Fasanmade A a, Wang B, Schaible T, Kavanaugh A, et al. The relationship of serum infliximab concentrations to clinical improvement in rheumatoid arthritis: results from ATTRACT, a multicenter, randomized, doubleblind, placebo-controlled trial. Arthritis and rheumatism. 2002 Jun;46(6):1451–9.
- Chang JT, Lichtenstein GR. Drug insight: antagonists of tumor-necrosis factor-alpha in the treatment of inflammatory bowel disease. Nature clinical practice Gastroenterology & hepatology. 2006 Apr;3(4):220–8.
- Colombel J-F, Loftus E V, Tremaine WJ, Egan LJ, Harmsen WS, Schleck CD, et al. The safety profile of infliximab in patients with Crohn’s disease: the Mayo clinic experience in 500 patients. Gastroenterology. 2004 Jan;126(1):19–31.
- Cominelli F. Cytokine-based therapies for Crohn’s disease--new paradigms. The New England journal of medicine. 2004 Nov 11;351(20):2045–8.
- Cornillie F, Shealy D, D’Haens G, Geboes K, Van Assche G, C
K 9654部分引文:
- Afif W, Loftus EV, Jr., Faubion WA, Kane SV, Bruining DH, Hanson KA, Sandborn WJ (2010). Clinical utility of measuring infliximab and human anti-chimeric antibody concentrations in patients with inflammatory bowel disease. Am J Gastroenterol 105(5): 1133-1139.
- Kopylov U, Mazor Y, Yavzori M, Fudim E, Katz L, Coscas D, Picard O, Chowers Y, Eliakim R, Ben-Horin S (2012). Clinical utility of antihuman lambda chain-based enzyme-linked immunosorbent assay (ELISA) versus double antigen ELISA for the detection of anti-infliximab antibodies. Inflamm Bowel Dis 18(9): 1628-1633.
- Tak PP (2012). A personalized medicine approach to biological treatment of rheumatoid arthritis: a preliminary treatment algorithm. Rheumatology 51(4): 600-609.
- Ordas I, Mould DR, Feagan BG, Sandborn WJ (2012) Anti-TNF monoclonal antibodies in inflammatory bowel disease: pharmacokinetics-based dosing paradigms. Clin Pharmacol Ther 91(4): 635-646.
- Bender NK, Heilig CE, Droll B, Wohlgemuth J, Armbruster FP, Heilig B (2007). Immunogenicity, efficacy and adverse events of adalimumab in RA patients. Rheumatol Int 27(3): 269-274.
K 9650部分引文:
- Afif, W. et al., 2010. Clinical utility of measuring infliximab and human anti-chimeric antibody concentrations in patients with inflammatory bowel disease. The American journal of gastroenterology, 105(5), pp.1133–9.
- Kopylov, U. et al., 2012. Clinical utility of antihuman lambda chain-based enzymelinked immunosorbent assay (ELISA) versus double antigen ELISA for the detection of anti-infliximab antibodies. Inflammatory bowel diseases, 18(9), pp.1628–33.
- Tak, P.P., 2012. A personalized medicine approach to biologic treatment of rheumatoid arthritis: a preliminary treatment algorithm. Rheumatology (Oxford, England), 51(4), pp.600–9.
- Ordás, I. et al., 2012. Anti-TNF monoclonal antibodies in inflammatory bowel disease: pharmacokinetics-based dosing paradigms. Clinical pharmacology and therapeutics, 91(4), pp.635–46.
- Bender, N.K. et al., 2007. Immunogenicity, efficacy and adverse events of adalimumab in RA patients. Rheumatology international, 27(3), pp.269–74.
看到这儿,您心动了吗?马上联系小艾吧!
或者扫描下方二维码,即可联系您的专属客服哦~

来源:Immundiagnostik英夫利昔单抗(Infliximab)药物浓度及药效检测试剂盒
作为一家具有高端的技术实力、先进的经营管理水平和完善的市场销售体系的生物高科技企业,总部位于武汉光谷高新技术开发区,服务面向全国。艾美捷科技是集进口试剂、实验室耗材销售、技术服务与合约开发为一体的专业化高科技公司,为用户提供专业的前沿资讯、完备的产品、整合的解决方案,及优质的物流服务。
艾美捷科技与国内外优秀的生物试剂供应商优保持着密切的合作关系,目前已成为众多国际知名品牌的中国总代理或一级代理,主要包括:AAT Bioquest、Abbexa、Abnova、Agrisera、Atlas Antibodies、BellBrook、Biomatik、Biosensis、BioVendor、CalBioreagents、Cayman Chem、Cell Biolabs、Columbia Biosciences、Crystal Chem、Cytoskeleton、DIAsource、Duchefa、Ebba Biotech、Echelon Biosciences、ECM Biosciences、Enzo Life Sciences、Epigentek、Equitech-Bio、FabGennix、G-Biosciences、GeneCopoeia、Hycult Biotech、ichorbio、Icosagen、Immundiagnostik、ImmunoReagents、IQ Products、Jackson、LC Labs、LifeSensors、Lumiprobe、Mabtech、Matreya、Medkoo Biosciences、MyBioSource、Nanoprobe、Norgen Biotek、ProSci、ProSpec、ReliaTech、Rockland、SouthernBiotech、StressMarq、SySy、TRC、US Biological、Worthington 等,可以在短时间内为用户提供专业的前沿资讯、完备的产品及物流服务。